TRYNGOLZA was studied to determine whether it was safe and effective for lowering triglycerides in adults with FCS
FCS=familial chylomicronemia syndrome.

FCS=familial chylomicronemia syndrome.
What was the main question (or primary endpoint) that the clinical trial of TRYNGOLZA was aiming to answer?
- All participants were assessed to understand how effective TRYNGOLZA had been at lowering their triglyceride levels compared with placebo at 6 months
What else did the study look at?
Select secondary questions included:
- Fasting triglycerides compared with placebo at 1 year
- Attacks of pancreatitis. Pancreatitis is inflammation of the pancreas that is extremely painful and potentially life-threatening
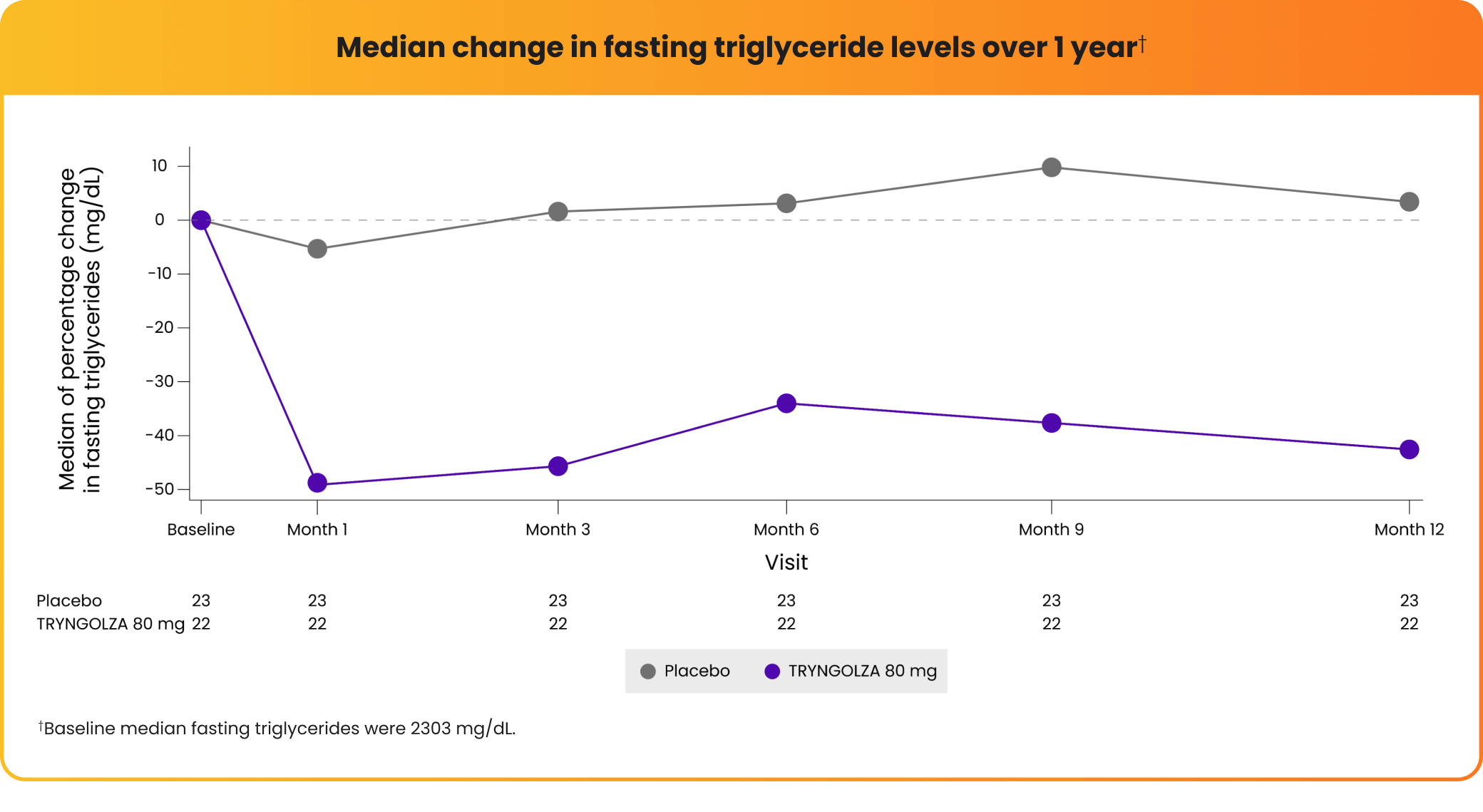
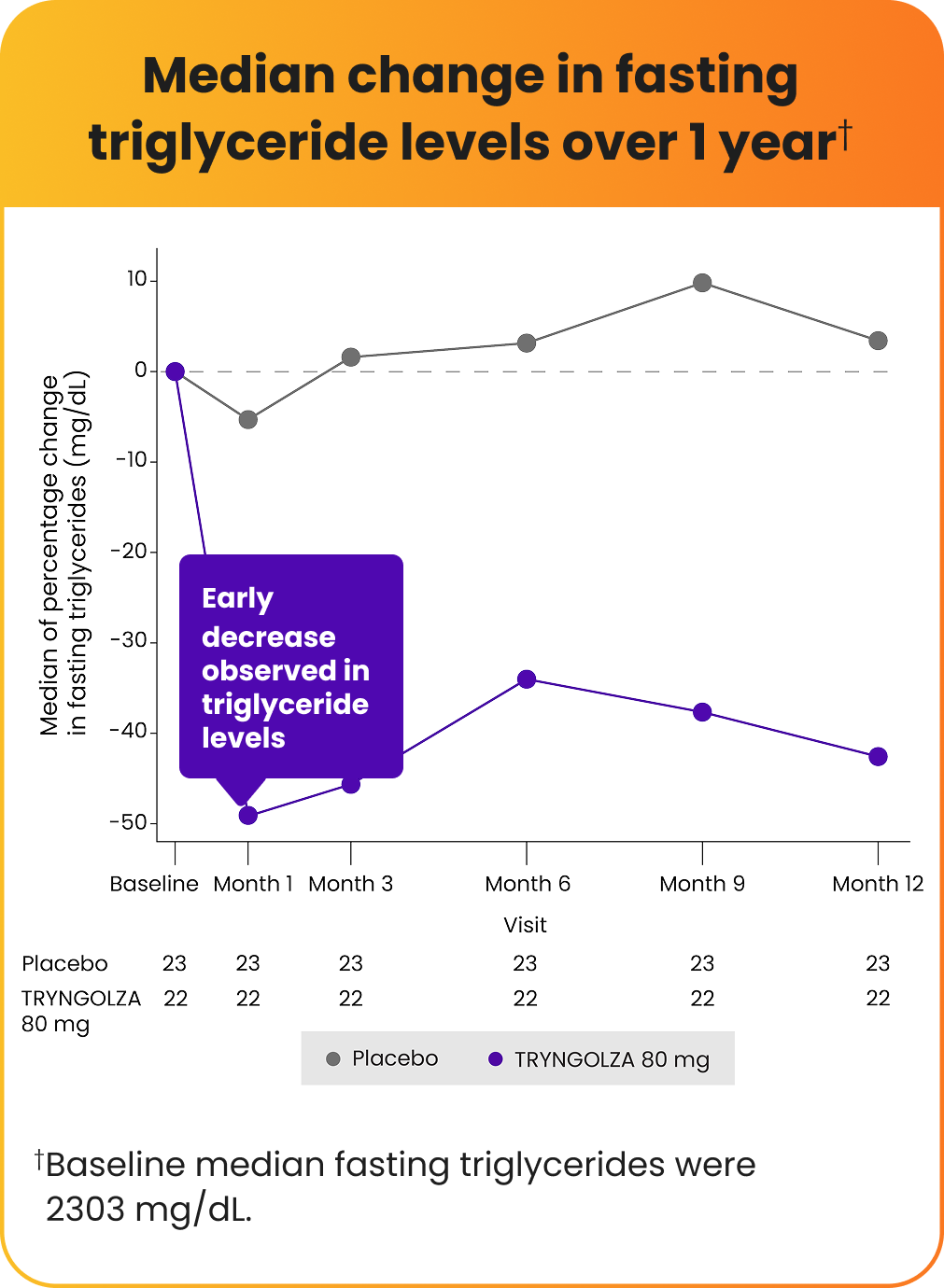
TRYNGOLZA significantly lowered triglyceride levels at 6 months, and levels continued to decrease over 1 year
In a clinical trial, the "mean" represents the average value of an outcome across all participants. The significant decrease in average triglyceride levels at 6 months, with continued reductions over 1 year, are shown below.
Main question

43%
average decrease in fasting triglycerides at 6 months compared with placebo*
Secondary question
57% average decrease in fasting triglycerides observed at 1 year compared with placebo*
*Baseline average fasting triglycerides were 2604 mg/dL.
In a clinical trial, the "median" represents the middle value when all data points are arranged in order. Consistent triglyceride lowering was observed over 1 year, as seen in median values below.
Fewer participants experienced attacks of acute pancreatitis over 1 year
There were fewer attacks of acute pancreatitis in participants taking TRYNGOLZA compared with placebo.
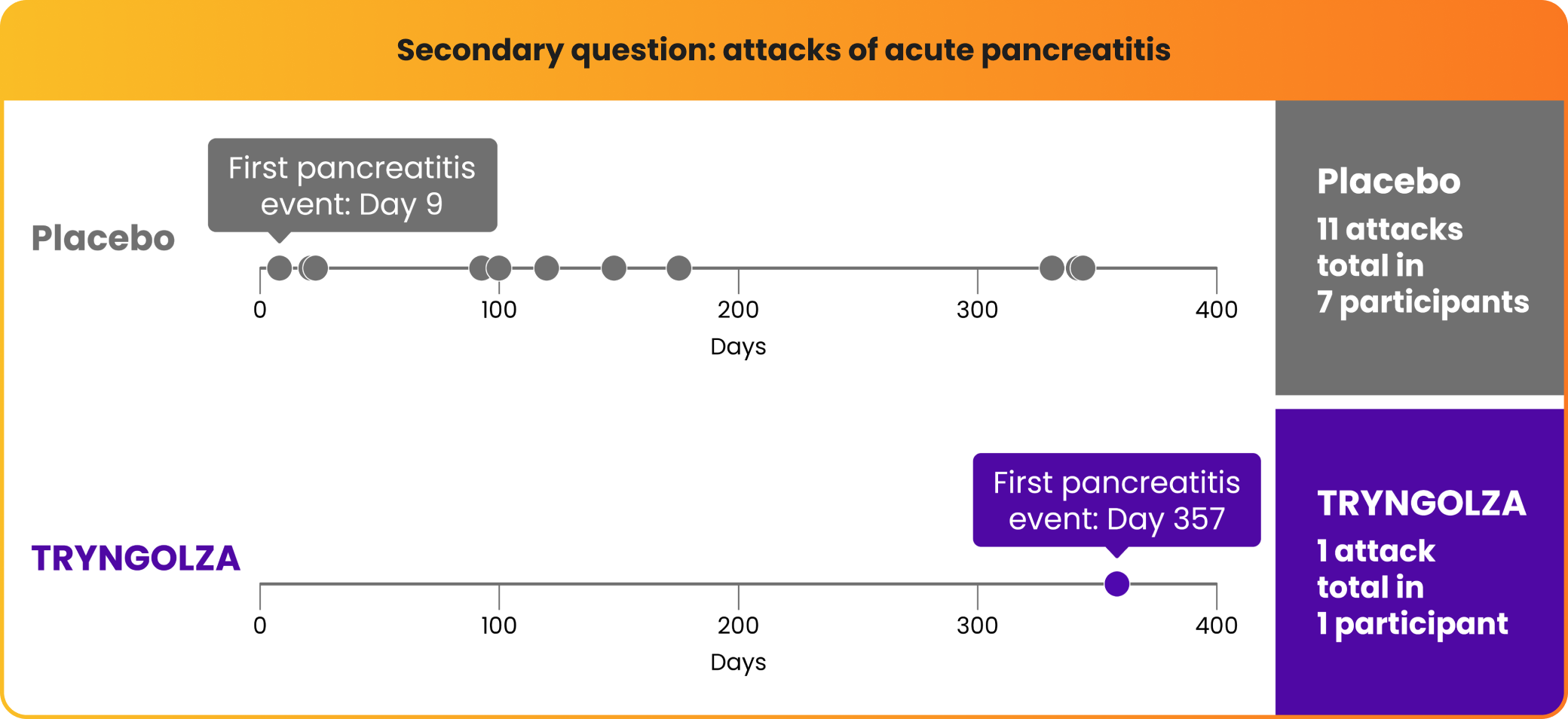
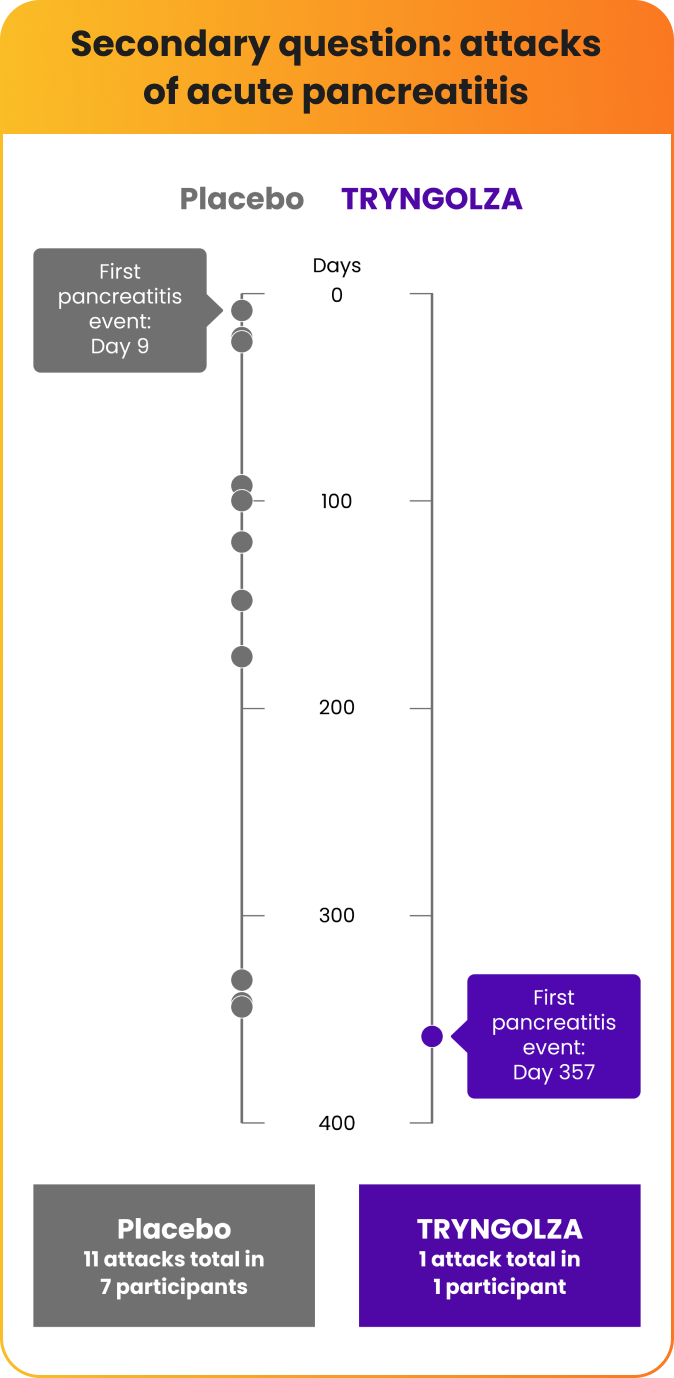
All of these participants had a prior history of pancreatitis within 10 years prior to screening.
Trial design





*A low-fat FCS diet was defined as ≤20 grams fat per day.
All the participants in the study:
Were 18 years and older
Had fasting triglyceride levels ≥880 mg/dL
Followed a low-fat FCS diet of ≤20 grams fat per day for at least 4 weeks prior to and during the study
Were identified to have a genetic variant known to be associated with FCS†
Patient demographic and baseline characteristics were generally similar among participants:
- 71% of all participants had a history of documented acute pancreatitis in the prior 10 years
- 32% in the TRYNGOLZA group had diabetes vs 26% in the placebo group
- Average fasting triglyceride level at baseline was 2604 mg/dL among all participants
- Most participants were taking stable doses of lipid-lowering treatments like statins, omega-3 fatty acids, and fibrates
None of the participants or healthcare professionals involved in the clinical trial knew who was being treated with TRYNGOLZA or taking the placebo (an inactive substance that looks the same as, and is given the same as, the active drug being studied). This is why the study is called a placebo-controlled, double-blind trial.
†The TRYNGOLZA Prescribing Information does not require a genetic diagnosis of FCS.
FCS=familial chylomicronemia syndrome.
